Document Type : CASE STUDY
Authors
Department of Civil Engineering, K. N. Toosi University of Technology, Tehran, Iran
Abstract
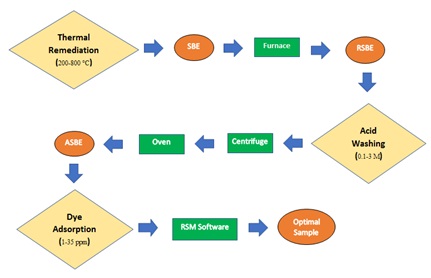
Bentonite bleaching earth is utilized for purifying used motor oil through a recovery process in order to improve the quality and stability of the final product. Indeed, spent bleaching earth is generated due to adsorbing oil impurities. Polluted spent bleaching earth contains 20-40% (w/w) oil and is flammable. Its disposal without pre-treatment leads to loss of oil along with environmental impacts. Accordingly, similar studies have been conducted since 1979 until now. This research was a laboratory study on reactive dye adsorption. Cleaning bleaching clay, thermal remediation and acid washing activation methods were utilized. Response surface methodology was used to design the experiments and determine the optimal parameters in order to run the dye adsorption process. The main experimental parameters have been concluded as temperature (200-800 °C), acid solution concentration (0.1-3 M), dye solution concentration (1-35 ppm), and ratio of activated earth to dye solution (0.1-2 %, w/w). Results revealed that dye adsorption process along with oil removal at a temperature of 650 °C, acid solution concentration of 0.83 M, dye solution concentration of 11.75 ppm and ratio of activated earth to dye solution of 1.52 % (w/w) results in an adsorption efficiency of 68.57%. This removal efficiency is a bit higher than activated virgin bleaching earth and much higher than virgin bleaching earth, which has adsorption capacities of 66.75% and 51.56%, respectively. Considering this recycling process, the purified material is quite acceptable technically, environmentally and economically.
Graphical Abstract
Highlights
- Dye adsorption followed by activated bleaching earth as a novel method of adsorbent SBE remediation
- Enhancing adsorption capacity around 20% in comparison with virgin bleaching earth
- Application of RSM acted to optimize the dye adsorption capacity
- Thirty optimum scenarios were selected through the "design expert software" and validated by the related experiments.
Keywords


Letters to Editor
[1] Letters that include statements of statistics, facts, research, or theories should include appropriate references, although more than three are discouraged.
[2] Letters that are personal attacks on an author rather than thoughtful criticism of the author’s ideas will not be considered for publication.
[3] Letters can be no more than 300 words in length.
[4] Letter writers should include a statement at the beginning of the letter stating that it is being submitted either for publication or not.
[5] Anonymous letters will not be considered.
[6] Letter writers must include their city and state of residence or work.
[7] Letters will be edited for clarity and length.
Send comment about this article