Document Type : REVIEW PAPER
Author
Artvin Coruh University, Engineering Faculty, Department of Environmental Engineering, Seyitler, Centrum, Artvin, Turkey
Abstract
Global warming is increasing permanently, because the concentration of CO2 in the atmosphere is rising continuously. According to National Oceanographic and Atmospheric Administration, the concentration of CO2 in the atmosphere was 407 ppm in June 2016 and 413 ppm in April 2017 as a last record for now. If the effects of other greenhouse gases, such as CH4, N2O, SF6, NF3, chlorofluorocarbons, hydrofluorocarbons, perfluorocarbons are added, the effective concentration may reach or exceed 550 ppm CO2-equivalent. According to the United Nations Intergovernmental Panel on Climate Change-2014 Climate Change Report, this is about two times higher than 278 ppm CO2 concentration in the pre-industrial year 1765. Thus, very urgent solutions must be found. The aim of this article is to suggest a vital, fast and very meticulous solution using NH3 gas in the atmosphere in order to decrease the atmospheric CO2 without delay. The laboratory experiments in the gas phase for (NH3+ CO2) reaction showed us that to use NH3 gas in the atmosphere will be a very fast, effective method for decreasing CO2 concentration of atmosphere. (NH3+ CO2) reaction is also quantitative in the cold atmosphere strata and there will be no more free ammonia in the atmosphere and no public health problem.
Graphical Abstract
Highlights
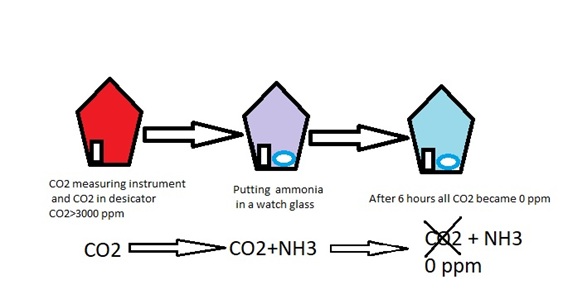
- The reaction of NH3 with CO2 to produce NH4HCO3 is a known reaction in industry
- The gas phase reaction of (NH3+CO2) was made in an empty glass desiccator
- After NH3 addition, the CO2 in desiccator became zero in about six hours
- It seems that NH3 spraying will be a very fast and effective method for decreasing CO2
- CO2 in the atmosphere transformed to NH4HCO3 as an N and C fertilizer.
Keywords


Letters to Editor
[1] Letters that include statements of statistics, facts, research, or theories should include appropriate references, although more than three are discouraged.
[2] Letters that are personal attacks on an author rather than thoughtful criticism of the author’s ideas will not be considered for publication.
[3] Letters can be no more than 300 words in length.
[4] Letter writers should include a statement at the beginning of the letter stating that it is being submitted either for publication or not.
[5] Anonymous letters will not be considered.
[6] Letter writers must include their city and state of residence or work.
[7] Letters will be edited for clarity and length.
Send comment about this article