Document Type : ORIGINAL RESEARCH ARTICLE
Authors
School of Environmental Engineering, Institute of Engineering, Suranaree University of Technology, Nakhon Ratchasima 30000, Thailand
Abstract
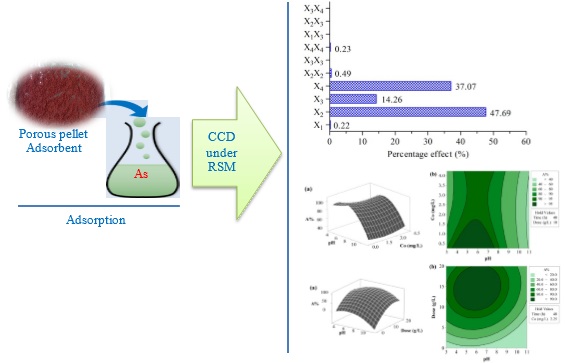
Mesoporous pellet adsorbent developed from mixing at an appropriate ratio of natural clay, iron oxide, iron powder, and rice bran was used to investigate the optimization process of batch adsorption parameters for treating aqueous solution coexisting with arsenate and arsenite. Central composite design under response surface methodology was applied for optimizing and observing both individual and interactive effects of four main influential adsorption factors such as contact time (24-72 h), initial solution pH (3-11), adsorbent dosage (0-20 g/L) and initial adsorbate concentration (0.25-4.25 mg/L). Analysis of variance suggested that experimental data were better fitted by the quadratic model with the values of regression coefficient and adjusted regression coefficient higher than 95%. The model accuracy was supported by the correlation plot of actual and predicted adsorption efficiency data and the residual plots. The Pareto analysis suggested that initial solution pH, initial adsorbate concentration, and adsorbent dosage had greater cumulative effects on the removal system by contributing the percentage effect of 47.69%, 37.07% and 14.26%, respectively. The optimum values of contact time, initial solution pH, adsorbent dosage and initial adsorbate concentration were 52 h, 7, 10 g/L and 0.5 mg/L, respectively. The adsorption efficiency of coexisting arsenate and arsenite solution onto the new developed adsorbent was over 99% under the optimized experimental condition.
Graphical Abstract
Highlights
- Optimizing the experimental conditions of the novel adsorbent developed from widely available and low cost materials to produce the maximum adsorption efficiency for arsenate and arsenite coexisting in aqueous solution
- A statistical method, known as the central composite design under response surface methodology has been applied for the optimization process
- Under batch observation, the model from the applied method is adequate and liable to provide significant agreement between the predicted and observed values leading to obtain the optimum value of the investigated factors
- The adsorption efficiency of the coexisting arsenite and arsenate solution onto the new developed adsorbent was over 99% under the optimized experimental condition.
Keywords
- Analysis of variance
- Arsenic removal
- Central composite design
- Mesoporous adsorbent
- Response surface methodology
Main Subjects


Letters to Editor
[1] Letters that include statements of statistics, facts, research, or theories should include appropriate references, although more than three are discouraged.
[2] Letters that are personal attacks on an author rather than thoughtful criticism of the author’s ideas will not be considered for publication.
[3] Letters can be no more than 300 words in length.
[4] Letter writers should include a statement at the beginning of the letter stating that it is being submitted either for publication or not.
[5] Anonymous letters will not be considered.
[6] Letter writers must include their city and state of residence or work.
[7] Letters will be edited for clarity and length.
Send comment about this article